
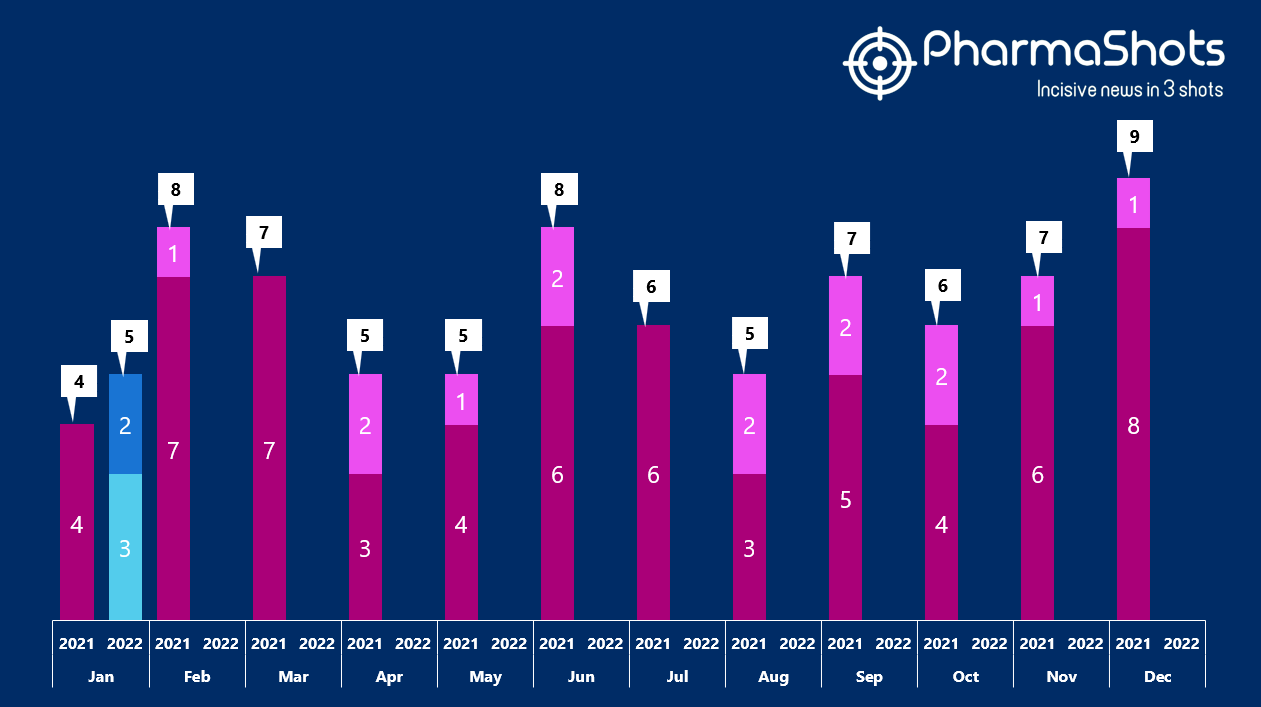
Insights+: The US FDA New Drug Approvals in January 2022
- The US FDA has approved 3 NDAs and 2 BLAs in Jan 2022, leading to treatments for patients and advances in the health care industry. The CDER and CBER approved 5 novel products in 2022
- In Jan 2022, the major highlights were Quviviq's approval for insomnia, Cibinqo for atopic dermatitis, Kimmtrak for unresectable or metastatic uveal melanoma, Vabysmo for neovascular (wet) age-related macular degeneration, and diabetic macular edema
- PharmaShots has compiled a list of a total of 5 new drugs approved by the US FDA in January 2022
Idorsia’s Quviviq (daridorexant) Receives the US FDA’s Approval for the Treatment of Insomnia
Quviviq
Active ingredient: daridorexant Approved: January 10, 2022
Company: Idorsia Disease: Insomnia
- The approval was based on the P-III clinical program i.e., Study 1&2 evaluate Quviviq (20/50mg) vs PBO in 1854 patients with insomnia at ~160 sites across 18 countries. The therapy is expected to be available in May 2022
- The results showed an improvement on objective measures of sleep onset, sleep maintenance & patient-reported total sleep time while 50mg dose in one of the 2 studies showed a reduction in patient-reported daytime sleepiness as measured by sleepiness domain score from IDSIQ @ 1& 3mos.
- The results for the 25mg dose did not reach statistical significance. Quviviq is a dual orexin receptor antagonist that blocks the binding of wake-promoting neuropeptides orexins
Ryaltris Nasal Spray
Active ingredient: mometasone furoate and olopatadine Approved: January 14, 2022
Company: Glenmark Disease: Seasonal Allergic Rhinitis
- The US FDA has approved the NDA for Ryaltris, a fixed-dose, aqueous suspension, prescription drug product nasal spray for the treatment of symptoms of seasonal allergic rhinitis in adults and pediatric patients aged ≥12yrs.
- Ryaltris will be available in the US by Hikma, following an exclusive license agreement with Glenmark & a recommended daily dose is 2 sprays, BID in each nostril. The product has been marketed in Australia, the Czech Republic, Poland, Russia, South Africa, Ukraine, Uzbekistan & the US
- The company has signed commercial agreements with multiple partners globally including Menarini to commercialize Ryaltris in the EU and with Bausch Health in Canada
Pfizer’s Cibinqo (abrocitinib) Receives the US FDA’s Approval for the Treatment of Atopic Dermatitis
Cibinqo
Active ingredient: abrocitinib Approved: January 17, 2022
Company: Pfizer Disease: Atopic Dermatitis
- The approval was based on the 5 trials i.e., JADE MONO-1/2, JADE COMPARE, dose-ranging trial & an ongoing OLE trial to evaluate the safety & efficacy of Cibinqo (100/200mg, qd) monothx vs PBO in 1600+ patients with AD whose disease is not adequately controlled with other systemic drugs
- The results showed improvements in skin clearance, severity & the extent of disease along with an improvement in itch @2wks. Additionally, a higher proportion of patients achieved an improvement in itching @12wks. & has a consistent safety profile
- Cibinqo is a JAK1 inhibitor & has received marketing authorization in the EU, Great Britain, Japan, Korea, the United Arab Emirates, Norway, Iceland, & Singapore
Kimmtrak
Active ingredient: tebentafusp-tebn Approved: January 27, 2022
Company: Immunocore Disease: Uveal Melanoma
- The approval was based on the P-III (IMCgp100-202) trial to evaluate Kimmtrak vs pembrolizumab, ipilimumab, or dacarbazine in a ratio (2:1) in 378 adult patients with mUM. The product is expected to be available in the US within wks.
- In a P-III trial, the patients treated with Kimmtrak showed an OS benefit with m-OS of 22mos., OS in the ITT population (82% pembrolizumab; 13% ipilimumab; 6% dacarbazine), treatment-related adverse reactions were manageable & consistent with the proposed mechanism
- The company has launched a KIMMTRAKConnect program to provide access to patients treated with Kimmtrak while BLA approval is currently under the RTOR program's review with an anticipated PDUFA date of Feb 23, 2022
Genentech’s Vabysmo Receives the US FDA’s Approval for the Treatment of AMD and DME
Vabysmo
Active ingredient: faricimab-svoa Approved: January 31, 2022
Company: Genentech Disease: AMD and DME
- The approval was based on the 4 P-III (TENAYA & LUCERNE) & (YOSEMITE & RHINE DME) studies evaluating faricimab vs aflibercept in 1329 & 1891 patients with wet AMD & DME. The product is expected to be available in the US in the coming wks.
- The results showed that patients treated with Vabysmo were given ~4mos. achieved non-inferior vision gains over aflibercept that administered q2mos. in the 1st yr., was well tolerated with a favorable benefit-risk profile
- Vabysmo is 1st FDA-approved injectable eye product for wet AMD & DME that improves & maintains vision while EMA is evaluating faricimab’s MAA for the same indication. The (COMINO) & (BALATON) trials are underway for macular edema
Related Post: Insights+: The US FDA New Drug Approvals in December 2021

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com














